Background: Myelofibrosis (MF) is a distinct subtype of the Philadelphia-negative myeloproliferative neoplasms (MPNs) characterized by an overproduction of differentiated hematopoietic cells. MF consisted of primary myelofibrosis (PMF) and secondary myelofibrosis (SMF) progressed from polycythemia vera and essential thrombocythemia. The pathologic and clinical features of MF include myeloproliferation, bone marrow fibrosis, anemia, splenomegaly, and MF-associated symptoms. But, some cases of MF shows cytopenic features, which were known to effect on inferior outcomes.
The previous studies evaluating the prognostic impact of cytopenia at diagnosis have a limitation because the progression of cytopenia through disease course cannot be reflected. Therefore, the study by a time-dependent model is required. In the present study, we evaluated prognostic implication of dynamic thrombocytopenia as a time-dependent variable in patients with myelofibrosis. And, we attempt to determine the genetic and immunologic factors associated with dynamic thrombocytopenia.
Methods: A total of 226 patients diagnosed with myelofibrosis and treated at Seoul St. Mary's Hematology Hospital from December 2001 to August 2021, who were performed DNA samples for next-generation sequencing (NGS) analysis were included in this study. To evaluated the correlation of various immune subsets and dynamic thrombocytopenia, immunophenotypic analysis by multiparameter flow cytometry was performed.
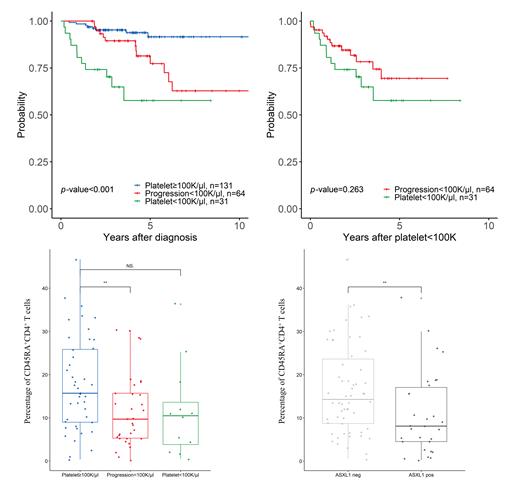
Results: The patients with myelofibrosis were composed of SMF (n=66, 29.2%), PMF (n=160, 70.8%). Among the patients, 100 × 10 9/L or more platelet count group (PLT≥100) was the most common (n = 131, 60%), followed by progression to thrombocytopenia (defined as a PROG) (n = 64, 28.3%), and less than 100 × 10 9/L platelet count group (PLT<100) (n = 31, 13.7%). We observed that the 4-year overall survival of patients was 57.7%, 89.4%, and 93.9% in the PLT<100, PROG, and PLT≥100 groups ( p<0.001), respectively. In the PLT≥100 groups, patients with PMF were fewer than SMF.
Next, we performed a time-dependent covariate analysis between the PLT≥100 group and PROG group. The progression of thrombocytopenia showed an inferior overall survival (P=0.004). In the multivariate analysis, the progression of thrombocytopenia ( p = 0.042, HR =7.7 [95% CI 1.04-7.70]), ASXL1 mutation ( p= 0.041, HR = 9.91 [95% CI 1.05-9.91]), and IDH1 mutation ( p =0.002, HR = 1103 [95% CI 5.19-1103]) were associated with poor OS.
The frequency of CD45RA +CD4 + T cells was lower in PROG group compared to the PLT>100 group ( p = 0.015). Among the patients in the PROG group, detection of ASXL1 mutation was higher than the other groups ( p=0.021). Genemic alteration of ASXL1 showed a trend for a decreased CD45RA +CD4 + T cells than counterpart ( p=0.01).
Conclusion: Our study demonstrated that dynamic thrombocytopenia, as a time-dependent variable has a prognostic value for the patients with MF, and low frequency of CD45RA +CD4 + T cells and genetic alteration of ASXL1 correlated with the progression to thrombocytopenia. Further investigation is warranted to determine the crucial clones and immune microenvironment signature associated with cytopenic features in patients with MF.
Disclosures
Kim:Abbvie: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Meiji Pharm: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Boryung Pharm Co.: Consultancy, Honoraria; Daiichi-Sankyo: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; AML-Hub: Consultancy, Honoraria; AIS biosicienc: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; BL & H: Research Funding; Takeda: Consultancy, Honoraria; Greencross Pharm: Consultancy, Honoraria; LG Chem: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal